Investigate renal drug transport modalities and drug interactions in vitro
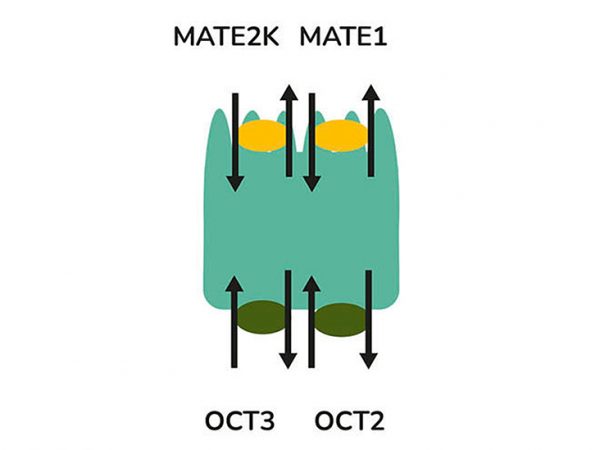
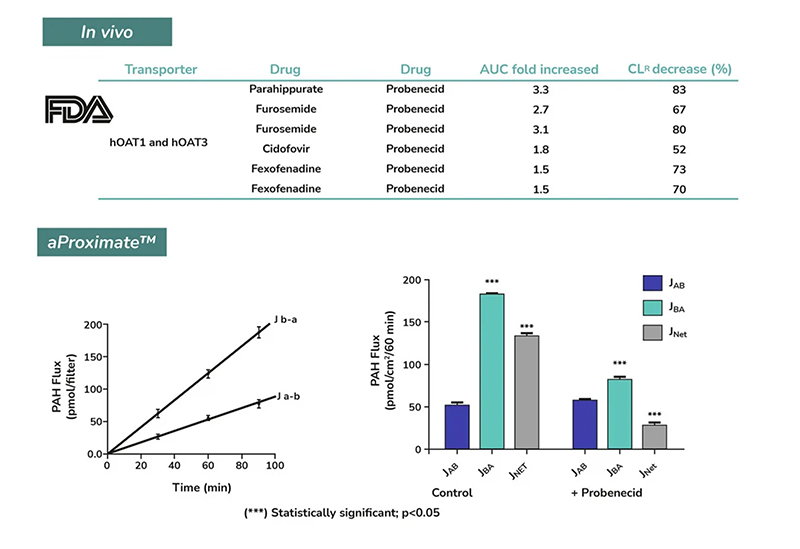
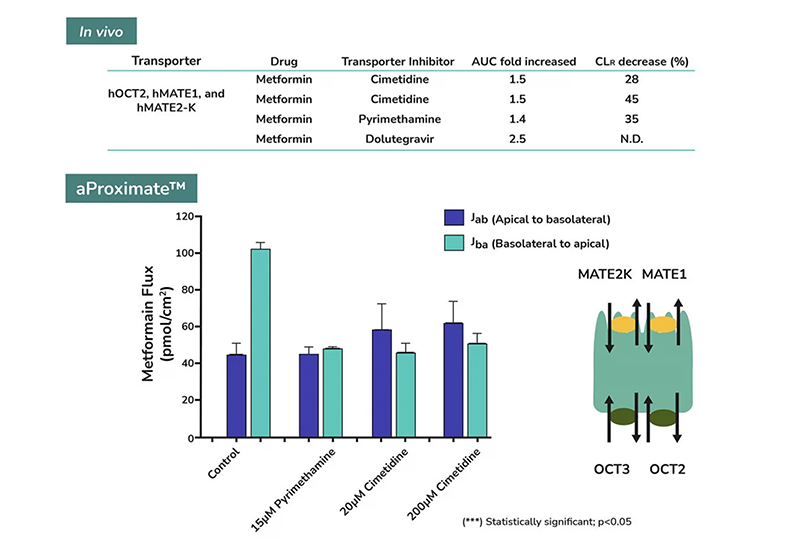
aProximate™ PTCs retain high expression of the relevant transporters involved in drug handling including megalin and cubilin; so are ideal for drug transporter and drug interaction studies of both small and large molecules. Transporters play a major role in the uptake and efflux of drugs across cellular membranes. Drug interactions with transporter proteins are common and can act either as substrates and/or inhibitors, a role which is best be identified during the early-stages of drug development to define the absorption, distribution, metabolism and excretion (ADME) profile. Using our scientific expertise, Newcells provides transporter assays using the aProximate™ model to support your specific requirements and understand potential drug interactions.
Service outputs
- Apical to Basal (Jab) and Basal to Apical (Jba) flux
- Net transport measurements
- Measurement of intracellular drug and metabolite concentrations
- Identification of transporter-mediated drug interactions
Don't miss out on our latest innovations: follow us on Linkedin