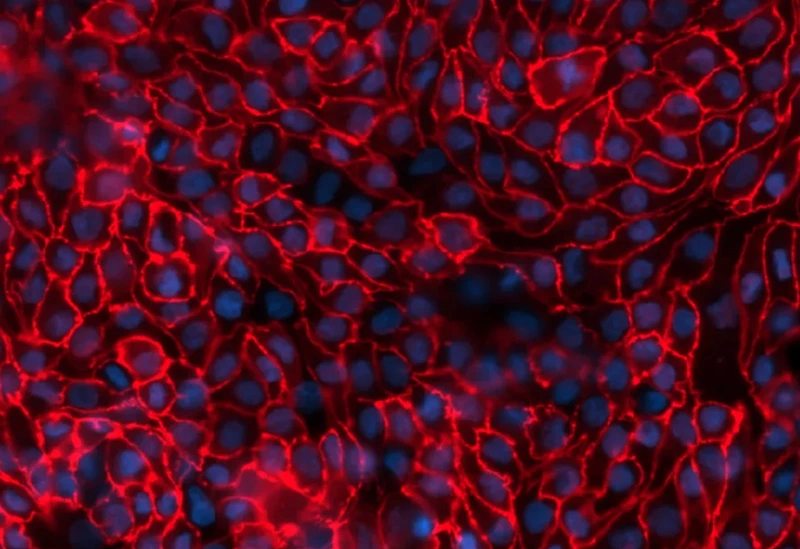
A unique service that speeds up anti-fibrotic therapeutics development.
Newcells’ FMT assay uses a validated high-throughput system and specialised culture conditions for human primary lung fibroblasts to provide robust data for research on respiratory disorders such as IPF.
Overview
Our FMT-assay improves the understanding of compound efficacy to advance anti-fibrotic drug-discovery programs.
This high throughput assay allows the study of fibroblast activation and collagen deposition in lung fibroblasts following stimulation with TGF-β.
The specialised set-up enables testing of small molecules and biologics for their ability to prevent fibroblast activation and ECM deposition across a range of concentrations.
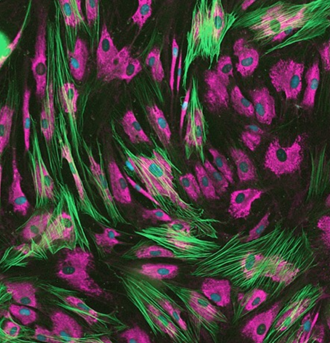
Newcells’ FMT assay replicates in vitro the changes occurring following lung injury, mimicking fibroblast activation and matrix deposition. The activation and transition of fibroblasts to myofibroblasts is characterized by the incorporation of α-smooth muscle actin (αSMA) into cellular stress fibres, promoting an increased synthesis and deposition of extracellular matrix (ECM) proteins, such as collagen I, to enable wound repair. Those are accurately quantified in our assay, making it the perfect tool to test novel anti-fibrotic compounds.
High-throughput Imaging
- Cell number
- Proliferation rates by (cellular EdU incorporation)
- Quantification of collagen I expression and deposition
- Quantification of α-SMA expression