Latest Advances in CAR-T Therapy with novel HLA-typed in vitro cell tools
In recent years, a ground breaking adoptive cell therapy known as Chimeric Antigen Receptor T-cell Therapy (CAR-T therapy) has emerged as a game-changer in the battle against cancer. This blog explores the progress of CAR-T therapy, its impact on cancer treatment, and the essential role of in vitro cell tools such as HLA-typed kidney cells of the proximal tubule and podocytes
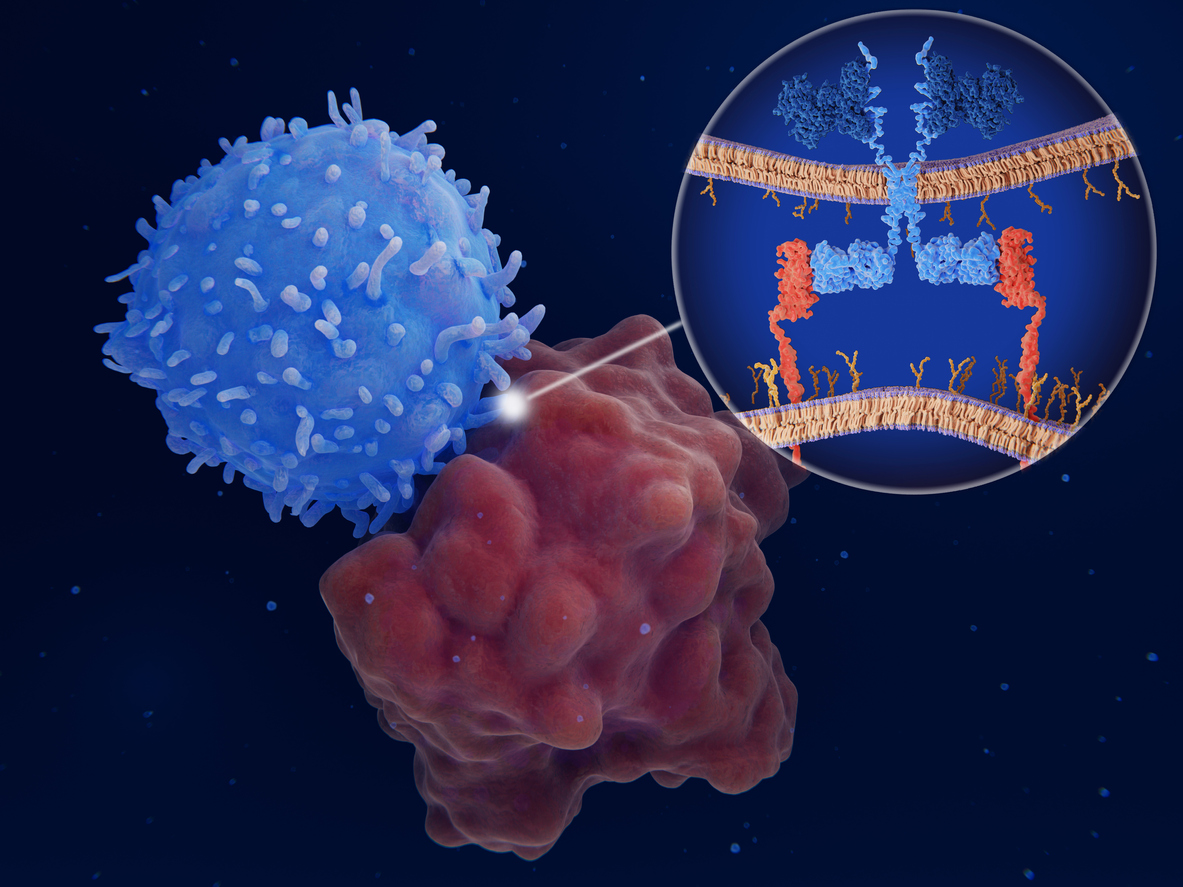
CAR-T Therapy
CAR-T Therapy is a form of immunotherapy that harnesses the power of the patient’s immune system to target and eliminate cancer cells. It involves ex-vivo genetic engineering of the patient’s T-cells with a viral vector to express chimeric antigen receptors (CARs) on their surface. These CARs are synthetic receptors that function to redirect lymphocytes (T-cells) to recognize and eliminate cancer cells expressing a specific antigen. Once the CAR-T cells are infused back into the patient’s bloodstream, they trigger vigorous T-cell activation and powerful anti-tumour response.
It took several decades from the concept of CAR-T therapy proposed in the late 1980’s by Dr. Zelig Eshhar, who envisioned a way to redirect T-cells to specifically recognise and attack cancer cells to the first FDA approved CAR-T therapy, in 2017, known as Kymriah (tisagenlecleucel), for the treatment of paediatric acute lymphoblastic leukemia (ALL) and unresponsive diffuse large B-cell lymphoma (DLBCL) in adults. This groundbreaking approval marked a turning point in cancer therapy, instilling new hope in patients and healthcare professionals alike. Additionally, CAR-T therapies such as Yescarta (axicabtagene ciloleucel) and Tecartus (brexucabtagene autoleucel) have expanded the scope of treatment showing significant efficacy in treating other types of aggressive lymphomas and leukaemia’s.
Overcoming Challenges and Advancements
CAR-T therapy has demonstrated ground-breaking success especially in treating haematological malignances, however, it also faces certain challenges. One of the most challenging drawback is cytokine release syndrome which varies from mild clinical symptoms such as fever and hypotension to life threatening toxicities and multi organ failure. Another hurdle is the development of resistance to CAR-T therapy over time leading to disease relapse. Researchers are actively investigating new strategies including the use of dual -targeted CAR-T cells and combination therapies.
The treatment of solid tumours also presents a challenge due to the tumour microenvironment preventing infiltration of CAR-T cells and the heterogeneity of target antigens presented on solid tumours. Ongoing research in the field focuses on CAR design, combination therapies with checkpoint inhibitors, AI and high -throughput approaches to improved current outcomes (Dagar et al., 2023),
The Role of In Vitro cell tools in CAR-T Therapy Research for rapid safety evaluation
Human HLA antigens have been associated with renal function, some being linked with an increased real function while other are associated with an increased risk of renal and chronic kidney disease, even though the exact nature of the link is unclear. (Lowe et al., 2021). It is therefore critical to evaluate response to new treatments in HLA -cell typed in vitro cell tools models for increased predictivity of human responses to new therapies. HLA -typed human Proximal tubule cells (PTCs) and podocytes provide optimal systems to test cancer reactive T-cells in culture to detect eventual cross reactions of novel CAR-T treatments and to accelerate kidney safety/toxicities assessments before human clinical trials. With HLA-typed cell models, scientist are also able to search for new antigens and biomarkers. Taken together, these in vitro cell tools provide valuable insights into the effects of CAR-T treatments on the renal system, aiding in the development of safer and more effective therapies.
Conclusion
CAR-T therapy’s ability to harness the specificity of adaptive immunity combined with its efficacy over time presents a powerful transformative approach to cancer treatment. With the ability to engineer the body’s own immune cells to specifically target and destroy cancer cells, CAR-T therapy has provided new hope for patients with previously untreatable cancers.
As research in CAR-T therapy continues to expand its application to various cancer types, HLA-typed kidney in vitro cell models such as human PTCs and podocytes constitute a precious tools for preclinical studies and lead compound validation and are essential in ensuring the safety and efficacy of CAR-T therapies.
With continued research and technological advancements, CAR-T cell therapy holds the promise of further revolutionising cancer treatment and improving the lives of countless patients.
References:
Dagar G, Gupta A, Masoodi T, Nisar S, Merhi M, Hashem S, Chauhan R, Dagar M, Mirza S, Bagga P, Kumar R, Akil ASA, Macha MA, Haris M, Uddin S, Singh M, Bhat AA. Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments. J Transl Med. 2023 Jul 7;21(1):449. doi: 10.1186/s12967-023-04292-3. PMID: 37420216; PMCID: PMC10327392.
Lowe, M., Payton, A., Verma, A. et al. Associations between human leukocyte antigens and renal function. Sci Rep 11, 3158 (2021). https://doi.org/10.1038/s41598-021-82361-7
Share on social media:
Don't miss out on our latest innovations: follow us on Linkedin
Jenny Cheung
29th August, 2023
Kidney
Update


