Accelerate in vitro preclinical evaluation of viral vectors for retinal gene therapy development
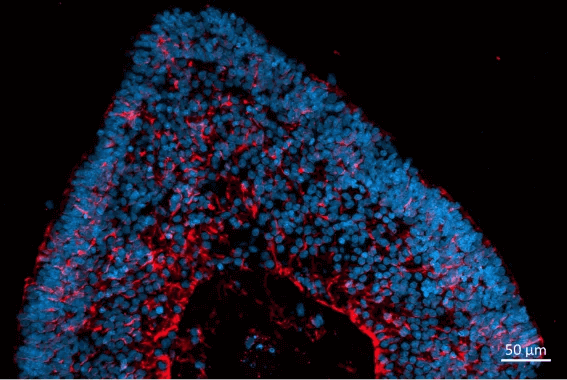
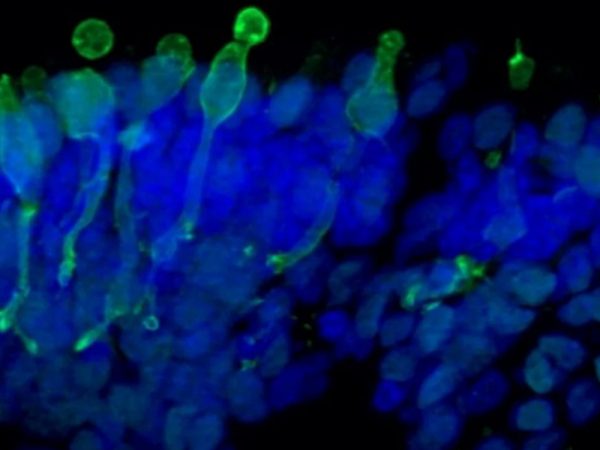
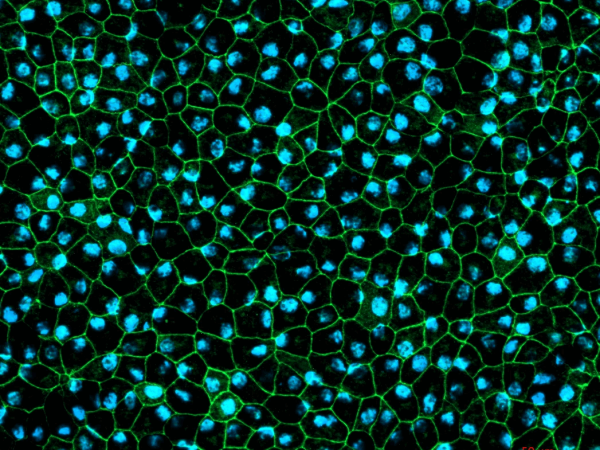
Newcells iPSC derived retinal organoids and RPE models are valuable tools for in vitro preclinical selection of new retinal gene therapy vectors.
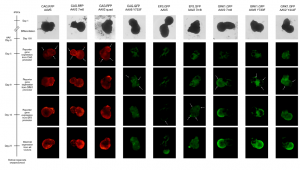
Gene therapy vector assessment service: This study allows for rapid in vitro evaluation of the best combination of vector capsid, promoter, and transgene and also enables initial safety and efficacy testing to be carried out using the exact same human promoter or gene as those to be used in clinical trials. Our human retinal models provide predictive data and have been validated for their use in screening and selection of optimal adeno-associated viral (AAV) vectors with increased photoreceptor tropism for retinal gene therapy.
Service outputs
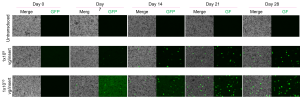
- Evaluation of AAV vectors transduction efficiency in iPSC-derived retinal organoids or RPE cells
- Analysis of AAV vector cell tropism and transduction of photoreceptor-like cells in retinal organoids and RPE
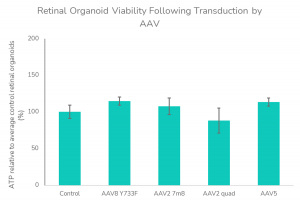
- Post-transduction cell viability assessment for safety prediction