DDI study on renal creatinine clearance mechanism
Tissue
Models of the Kidney NephronModels and Services
Drug Transporter Interactions & DDI studyThe Problem of the Client
The client wanted to characterise the creatinine transport mechanism in human Proximal Tubule Cells (PTC) models (both HEK293 and primary). Recent studies had suggested a potential involvement of Organic Anion Transporter 2 (OAT2) in addition to Organic Cation transporter 2 (OCT2) in creatinine clearance which therefore led to a requirement for additional drug-drug interaction (DDI) studies during drug development.
Solution
In collaboration with the team at Pfizer, we carried out an in vitro study using the human aProximateTM Proximal Tubule Cells model to understand the creatinine transport mechanisms.
Step 1:
Understanding the Problem
Measurement of creatinine clearance is correlated with the function and inhibition of the OCT2/MATE transporter in vivo. Our team needed to design a specific study that would evaluate the transport of creatine through the OCT2/MATE transporter. The study needed to be based on a human relevant PTC model with functional OCT2/MATE transporter and would form the basis of a subsequent study to examine the effect of a set of ASOs on the creatinine flux.
Step 2:
Developing a Customised Experimental Plan to Compare 3 ASOs in the Human PTC Model
The study included 3 parts:
- Preparation of freshly isolated Proximal Tubule Cells model from a healthy human kidney.
- Creatinine Transporter study including transepithelial flux in the presence and absence of inhibitor.
- Statistical data analysis.
Step 3:
Project Execution within 3 Months
Our scientists delivered on the agreed experiments within 3 months providing regular updates to the customer.
The study included the experimental phase, data processing, data analysis and presentation of a comprehensive data summary.
Output Dataset:
The study is published in the journal of Pharmacology and experimental therapeutics (read here).
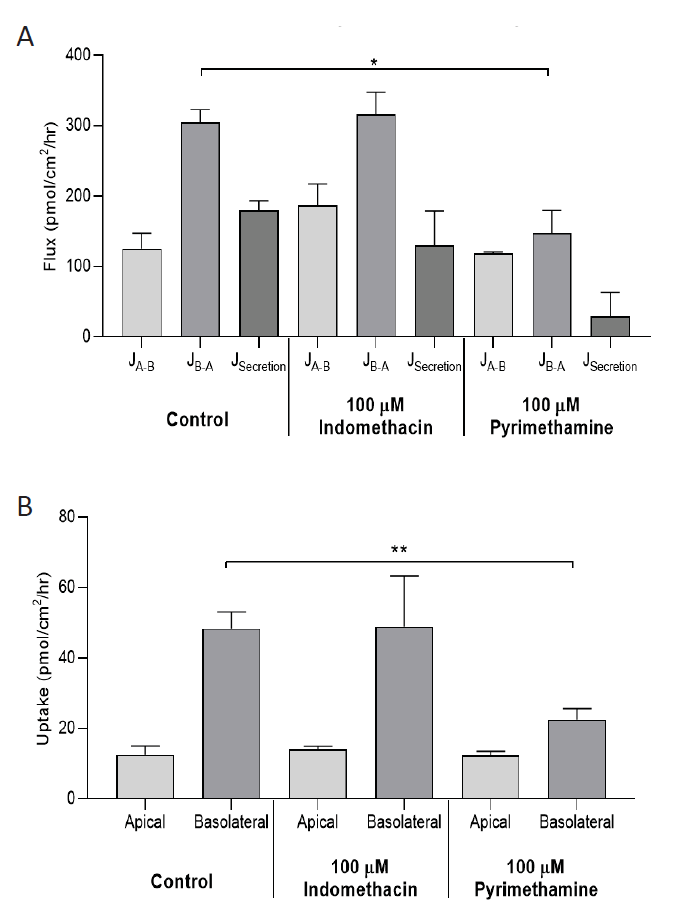
Data analysing bidirectional flux and accumulation of creatinine, in the absence and presence of indomethacin and pyrimethamine, was evaluated in hPTC model aProximateTM.

Effect of indomethacin (100μM) and pyrimethamine (100μM) on transcellular flux (A) and cell accumulation (B) of creatinine in hPTCs. Apical-to-basolateral (JA-B) and basolateral-toapical (JB-A) transport was measured in confluent monolayers with 10μM creatinine on the donor side, and cellular accumulation was measured at the end of incubation (30min). Secretory flux (JSecretion) is the difference between JB-A and JA-B. Data represent mean±SD from three independent experiments. * p<0.05, **p<0.01, significantly different from control. Paired one tailed Student’s t-test.
Step 4:
Delivery of Results
We delivered a detailed report of the dataset which was shared digitally and discussed with the customer. The study highlighted:
- The advantages of using freshly isolated hPTCs to quantify creatinine uptake enabling the functional assessment of renal transport mechanisms.
- That OCT2/MATE transporters play a major role in the active renal secretion of creatinine, while OCT2 does not.
Outcomes for the Client and the Project
These findings enabled the client to assess functionality of the OCT2/MATE transporter and conclude that the OCT2/MATE transporter is crucial in the transport of creatinine and quantitative pharmacokinetic data from it should be obtained when evaluating serum creatinine and creatinine clearance modulation in DDI studies. This approach would reflect more accurately the primary mechanisms involved in creatinine transport and clearance, leading to more reliable predictions of drug interactions and their effects on renal function markers. The study is published here.