Molecular and cellular mechanisms of kidney disease modelling in vitro
Kidney disease modelling service
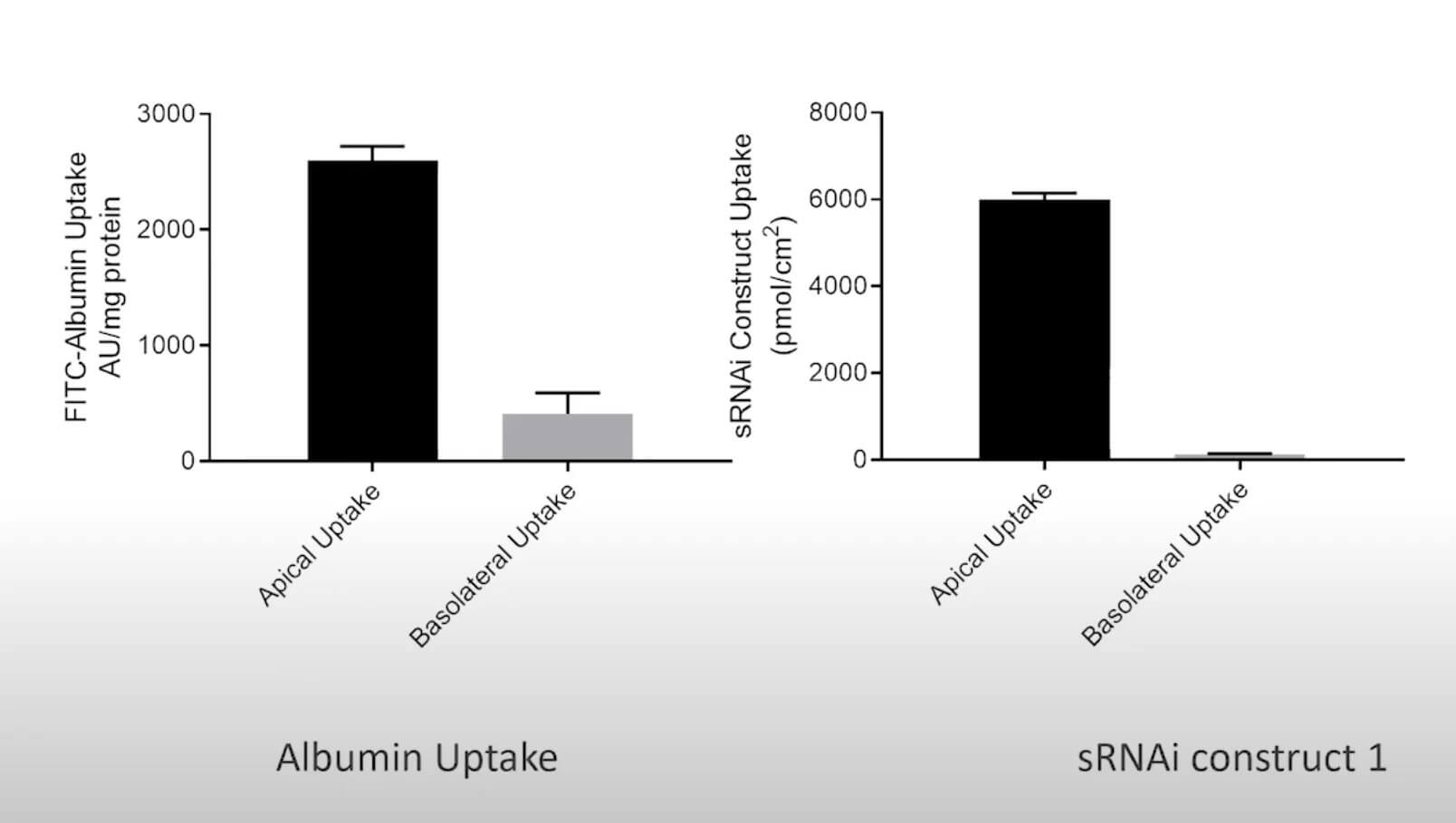
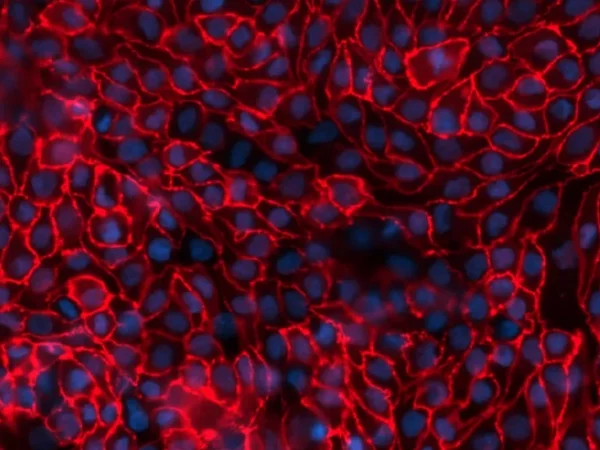
The Proximal Tubule aProximate™ model has been used to study a range of diseases including chronic kidney disease (CKD), gout, and metabolic disorders. To study renal dysfunction and perform in vitro kidney disease modelling, a robust kidney model, such as aProximate™, expressing all the transporters specialised in handling of drugs, solutes, and proteins is required. The Newcells aProximate™ model utilised in this service is unique as it is highly predictive of in vivo outcomes and includes kidney organoids to mimic how cells behave in the body. It also allows insights into disease mechanisms and assessment of therapeutic targets (e.g., renal transporters) of new drugs.
Service outputs
-
aProximate™ PTC model
Renal transporter expression, high throughput screening of drug modulators for specific transporter, Flux assays