Predictive kidney toxicity & drug safety evaluation, in vitro
Drug-induced nephrotoxicity is one of the leading causes of drug failure, and the prediction of kidney toxicity during new drug development remains inaccurate. Early detection of in vitro nephrotoxicity is key to reducing both costs and timelines of drug development. Advanced kidney toxicity assays, often known as nephrotoxicity assays, are able to evaluate kidney toxicity using human-relevant kidney toxicity models, provide essential data and offer insights to improve drug safety. These kidney toxicity assays provide precision nephrotoxicity testing to understand kidney function.
Generation biomarkers are used for detecting kidney toxicity through nephrotoxicity testing.
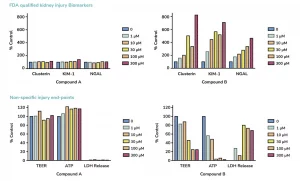
The Food and Drug Association (FDA) recommends in vitro precision nephrotoxicity assessment of new drugs and biologics prior to in vivo assessments in animal models, including human, rat, mouse and dog. Additional regulatory guidance from both the FDA and European Medicines Agency (EMA) also recommends the detection of early markers of kidney toxicity such as KIM-1, NGAL and Clusterin, which can only be quantified in more complex in vitro kidney models. Such nephrotoxicity models are therefore needed for reliable predictive and early evaluation of renal safety profiles.
Newcells advanced kidney in vitro nephrotoxicity assays allow researchers to better predict renal safety profiles earlier in the drug development process. Since kidney toxicity, or nephrotoxicity, can lead to kidney injury, it is important that these assessments are carried out at this stage. All kidney toxicity studies are run on Newcells aProximate™ Proximal Tubule Cell (PTC) model, which is an advanced MPS model containing all relevant biomarkers and is designed to assess renal toxicity with unparalleled accuracy. Designed to assess kidney toxicity with unparalleled accuracy, these nephrotoxicity models enable the detection of early and late toxic effects, fulfilling a critical need in the field of in vitro nephrotoxicity assessment through the detection of early nephrotoxic markers and transporter activity.
BASIC Nephrotoxicity study: this service rapidly assesses renal toxicity in species including human, mouse, rat or dog through quantification of cellular viability (ATP and LDH levels) and trans-epithelial electrical resistance (TEER).
COMPREHENSIVE Nephrotoxicity study: this service enables in-depth assessment of renal toxicity in species like human, mouse, rat or dog. The kidney toxicity assays include quantification of FDA-approved kidney injury markers NGAL, KIM1 and Clusterin, ensuring your IND submissions will be further strengthened by the testing of parameters and biomarkers that follow regulatory guidelines.
Gene analysis, inhibitor study and cross species comparison (human, mouse, rat and dog) are available as insightful complements.
Service outputs
- Assessment of FDA-approved biomarkers of kidney toxicity: KIM-1, NGAL and Clusterin
- Cell viability: ATP, LDH and TEER measurements