Development and characterisation of an early-stage retinal organoid disease model (for non-ocular disease)
Tissue
Retina ModelsThe Problem of the Client
We designed a study to differentiate retinal organoids from iPSCs containing the mutation of interest and compared them to organoids derived from isogenic wild type (WT) iPSCs.

Solution
We designed a study to differentiate retinal organoids from iPSCs containing the mutation of interest and compared them to organoids derived from isogenic wild type (WT) iPSCs.
Step 1:
Understanding the Problem
We defined the problem and questions to be answered through in-depth discussion between the customer and our technical experts.
The study required the development of isogenic wild type and diseased retinal organoids. Newcells’ proprietary technology could be used for the differentiation of WT and gene-edited iPSCs supplied by the client into retinal organoids. As the client was interested in early stages of retinogenesis, it was necessary to use and analyse the structure of the organoids via bright and fluorescent imaging at early time points of differentiation.
The organoids recapitulate the neural retina and the client also requested the shipping of the disease model organoids at day 60 and 90 of differentiation.
Step 2:
Developing a Customised Experimental Plan
We designed a customised study based on 4 steps and presented the plan to the client through our Statement of Work:
- Expansion of iPSCs and preparation of cell stocks.
- Generation of retinal organoids using a proprietary differentiation process and analysis of the retinal organoids at 30-, 60- and 90-days post-differentiation.
- Morphological characterisation and identification of neural retina and RPE within the retinal organoids.
- Qualitative immunofluorescence of photoreceptor markers (RCVRN and CRX) and of retinal ganglion cell marker (BRN3A).
Step 3:
Project Execution
Our scientists carried out the agreed experiments providing regular updates to the customer.
The experiments used brightfield and qualitative immunofluorescence to compare morphology and the presence of key retinal cell types in the disease model organoids with isogenic WT controls and positive control WT organoids at 0-, 30-, 60- and 90-days of differentiation.
The study included the experimental phase, data processing, data analysis and presentation of a comprehensive data summary.
Output Data Set:
Brightfield images were taken at each time points (n=3 per line) with an EVOS imaging system (Thermo Fisher).
Morphology scoring and identification of neural retina and RPE was performed by plate scans at each time point.
Qualitative immunofluorescence analysis of retinal markers was performed at day 60 of differentiation (12 organoids per condition and 3 images per line).
Example data of organoid morphology scoring:
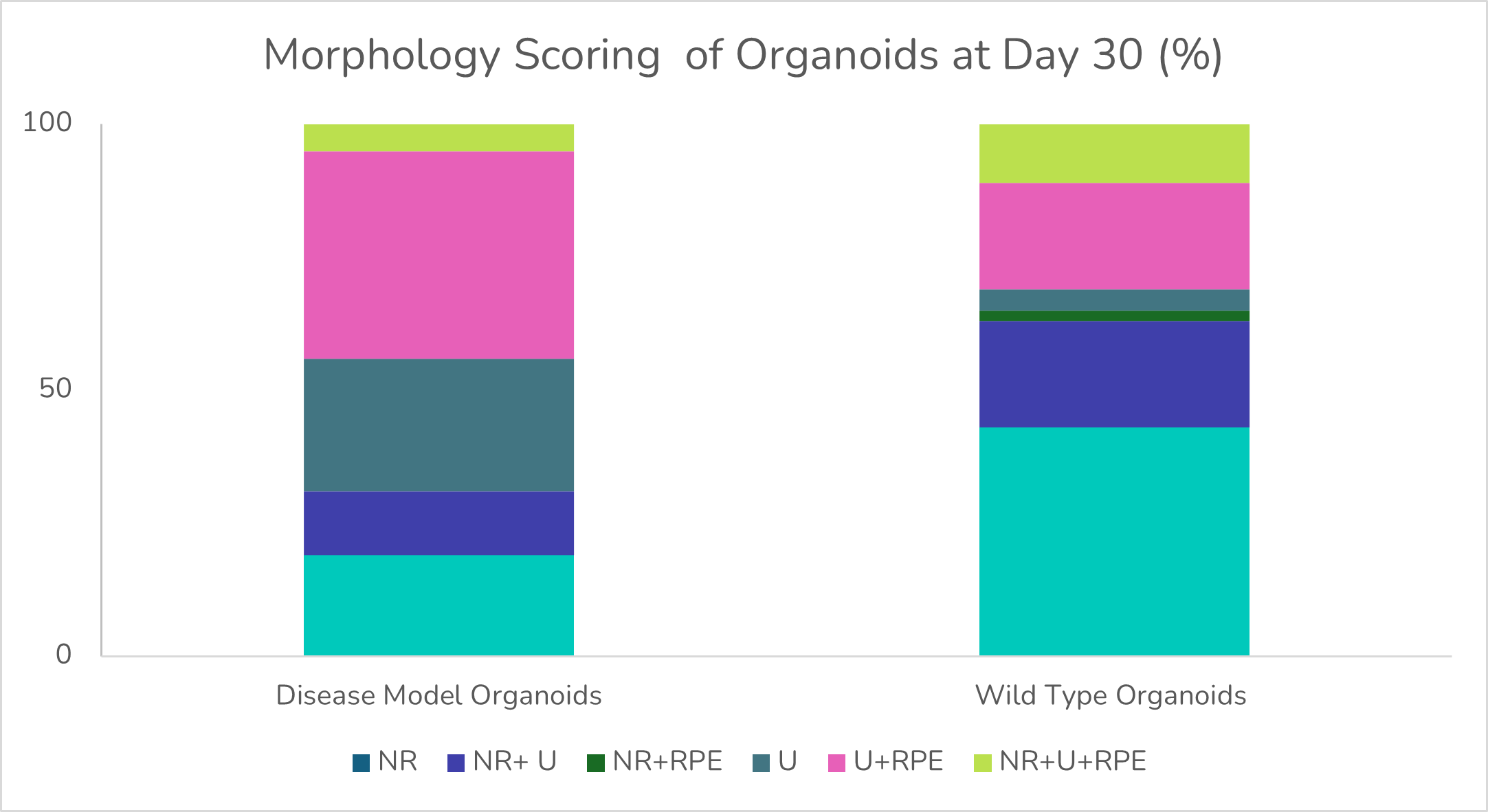
The morphology scoring analysis determined the percentage of neural retina (NR) and retinal pigment epithelium (RPE), with (U) corresponding to undefined areas.
Step 4:
Delivery of Results
We delivered a detailed report of the dataset which was shared digitally and discussed over a call.
The study showed:
- The disease model retinal organoids contained less neural retina areas than their WT counterparts at day 30 of differentiation, decreasing further at later timepoints.
- The disease model organoids expressed all key retinal markers at day 60 of differentiation without clear NR regions and less structural organisation than their WT counterparts.
- The disease model organoids displayed retina pigment epithelium.
Outcomes for the Client and the Project
The client acquired predictive human data showing their specific mutation for a non-ocular disease impacts retinogeneis in a human in vitro retinal organoids model.