Safety and efficacy evaluation of a novel therapeutic AAV vectors using a human retinal organoid model
Tissue
Retina ModelsThe Problem of the Client
The client wanted to acquire additional data prior to progressing a novel AAV vector to preclinical development stage. More specifically, the client wanted to determine the transduction efficiency of an adeno-associated virus (AAV) vector in human retinal organoids and assess the safety of this vector.

Solution
Step 1:
Understanding the Problem
Through in-depth discussion between the customer and our technical experts, we designed a study to assess the safety and efficacy of a novel AVV vector using retinal organoids.
We determined that the study should aim to measure AAV transduction efficiency as well as cell viability and toxicity following transduction of the disease relevant retinal organoids.
Step 2:
Developing a Customised Experimental Plan
We designed an extensive study starting with the development of disease model retinal organoids at day 120, 150 and 180 of differentiation and transduced them with two doses of AAV.
The study readouts included live cell imaging, immunofluorescence, cytotoxicity assay, cell viability, and transduction efficiency analysis by flow cytometry. The client also wished to receive organoid pellets for additional in-house testing.
Step 3:
Project Execution within 3 Months
The full project was carried out by our scientists within 3 months during which the client was provided with regular updates. The study included the experimental phase, data processing, data analysis and presentation of a comprehensive data summary.
Experimental readouts were acquired from 7 to 28 days post -transduction and included brightfield and fluorescent imaging of live cells. The toxicity study included ATP quantification, LDH release, flow cytometry and immunofluorescence analysis. The experiments were carried out in triplicate with pools of 10 retinal organoids for each readout. Three time points (120, 150 and 180 days ) of retinal organoid differentiation were tested for the control vector and for the test vector, each at two concentrations; requiring 150 organoids per time point.
Example of resulting data set:
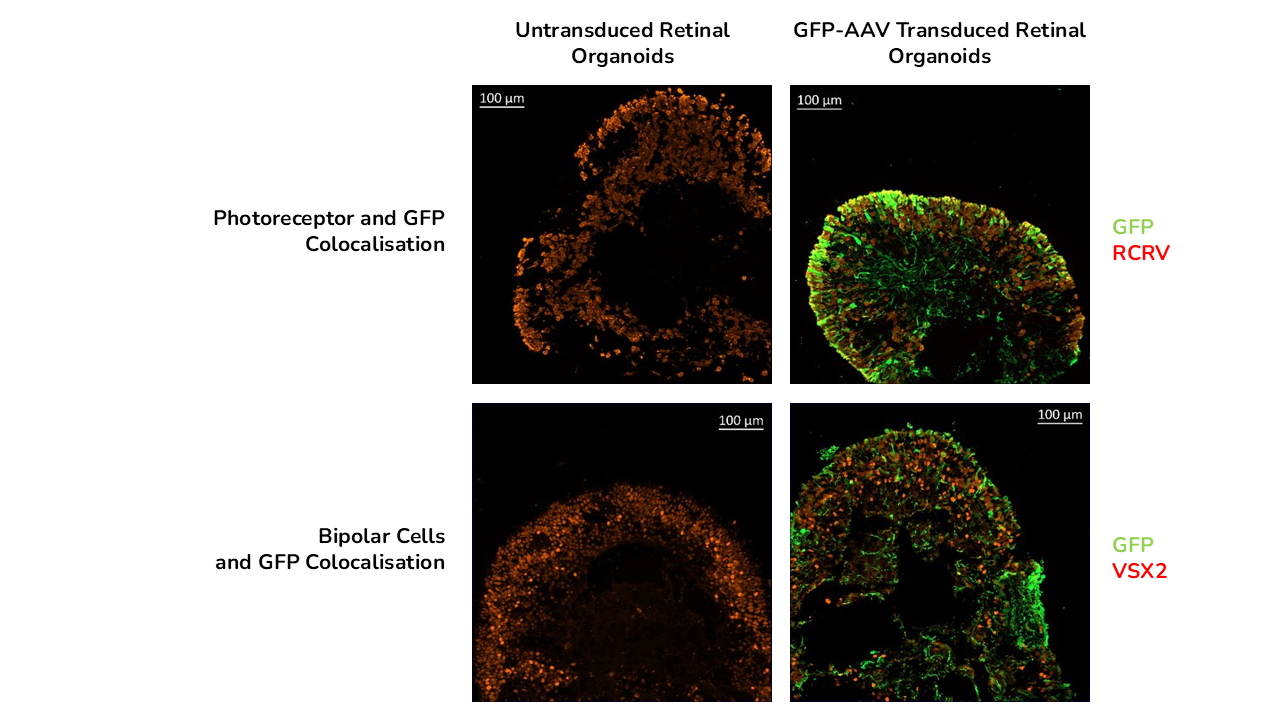
Immunofluoresence colocalization study at day 150 of GPF and Photoreceptor marker RCVRN at day 150 but did not colocalise with bipolar cell marker VSX2.
The study showed that viral vector transduction was more efficient at the higher AAV dose, showing mostly transgene (GFP) expression in the neural retina layers of the retinal organoids. The study also showed cellular localisation of the vector with GFP staining colocalised with photoreceptor marker RCVRN at day 150 and not with bipolar cell marker VSX2.
Step 4:
Delivery of Results
We delivered a detailed report of the dataset which was shared digitally and discussed with our client over a call.
The study clearly showed that:
- The tested AAV vector efficiently transduced the neural retina of the retinal organoids at all time points tested.
- Transduction efficiency was increased with the higher dose of viral vector and increased over time.
- No significant toxicity was observed post-transduction in day 150 and day 180 retinal organoids, but cytotoxicity was detected at day 120.
- Transgene expression was colocalised with the photoreceptor layer in human retinal organoids including rods and cones but not in other cell types such as bipolar cells.
Outcomes for the Client and the Project
The data presented confirmed that iPSC-derived ROs can be efficiently transduced with novel AAV gene therapy vector, it gave predictive information about the safety of the vector and subsequent confidence to progress the vector to preclinical in vivo studies.