Gene therapy viral vector assessment on RPE cells
Tissue
Retina ModelsThe ask
Our customer‘s goal was to confirm the dose-dependant ability of AAV constructs to transduce retinal tissue, more specifically the retinal pigment epithelium (RPE).

Our approach to developing a solution
We followed our standardised series of steps to develop a customised solution.
Step 1:
UNDERSTANDING THE PROBLEM
We clearly defined the problem and questions to be answered through in-depth discussion between the customer and our technical experts.
The customer wished to rapidly evaluate if their AAV vector was able to transduce RPE cells and if the transduction efficiency was dose-dependant.
Taking all project specific factors into account, we suggested a two-step approach as being the most appropriate for their situation:
- Step 1: Optimise the transduction of RPE with AAV vector
- Step 2: Image quantification and flow cytometry to quantify transduction efficiency
Step 2:
DEVELOPING A CUSTOMISED EXPERIMENTAL PLAN
We designed the specific experiments and presented the plan in our Statement of Work.
Our experimental plan involved using fully validated human iPSC-derived RPE.
For Step 1: RPE transduction
- We transduced human RPE with AAV vector containing a GPF reporter gene and encoding for AAV5 capsid and monitored transgene expression with live imaging up to 27 days post-transduction
- The experiments aimed to evaluate the transduction efficiency of AAV constructs at two concentrations
For Step 2: Imaging analysis
- Quantification of the % of GFP-positive cells
- Quantification of the transduction efficiency of AVV vectors in RPE by flow cytometry
Step 3:
PROJECT EXECUTION
Our scientists carried out the agreed experiments within 3 months providing regular updates to the customer.
The study included the experimental phase, data processing, data analysis and presentation of a data summary.
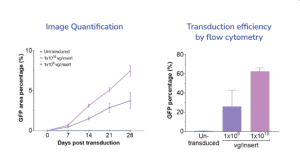
Example dataset
Step 4:
RESULT DELIVERY
We delivered a detailed report with reliable dataset which was shared digitally and discussed over a call upon request.
The study clearly showed :
-
Percentage of the area with GFP-positive cells over 28 days post transduction increased over time and in dose- dependent manner
-
Flow cytometry analysis allowed for accurate quantification and confirmed the transduction efficiency of RPE with AAV was dose- and time-dependant.
iPSC-derived RPE cells transduction study confirmed efficiency of AAV5 gene therapy vector to progress preclinical and in vivo studies.
Outcomes for the client
We delivered a robust dataset which clearly showed that human IPSC-derived RPE cells are a robust in vitro model for testing of AAV constructs. Our analysis gave confidence to our customer to proceed to preclinical studies with their AAV construct.