Predicting renal retention of radioconjugates in a human relevant in vitro model
Tissue
Models of the Kidney NephronThe Problem of the Client
The kidney is one of the main organs at risk for toxicity from radiopharmaceutical therapy, with 5% complication rate such as clinical nephropathy within 5 years. Our client needed to evaluate possible renal retention of radioconjugates and for this required an in vitro model that would allow them to not only detect early nephrotoxicity but also late renal toxicity often seen as cumulative, chronic and progressive in patients.

Solution
Exploiting the unique properties of the aProximateTM model to measure flux and retention of a radioconjugate peptide in vitro.
Step 1:
Understanding the Problem
We defined the problem and questions to be answered through in-depth discussions between the client and our technical experts.
Radioconjugates are used for targeted radiotherapy in cancer as a promising alternative drug modality to avoid the damage to heathy cells during external radiation therapy.
By linking radioisotopes to molecules capable of targeting tumour cells, it is possible to deliver high doses of ionising radiation to kill cancer cells with greater specificity.
The radioconjugates contains four parts: a chelating agent attached to a radioactive isotope and a linker connected to targeting compounds such as a peptide (linear or bicyclic), an antibody (mono and bispecific), or even and antibody drug conjugate (ADC).
We needed to design a study that would allow to determine renal accumulation of radioconjugates in rat kidney proximal tubule cells and identify the transporters involved.
Step 2:
Developing a Customised Experimental Plan to Compare 3 ASOs in the Human PTC Model
We designed a study to include:
- Rate and kinetics of uptake of peptide in primary rat proximal tubules
- Measurement of intracellular accumulation
- Accumulation kinetics study
- Inhibitor study to identify the renal transporters involved
Step 3:
Project Execution within 3 Months
Our scientists delivered the study within the agreed timeline providing regular updates to the client.
The study included the experimental phase, data processing, data analysis and presentation of a comprehensive data summary.
Each experiment and each data point were done in triplicate and repeated with at least 2 donors .
The uptake of the radioconjugate was measured at the relevant timepoints and in both directions (Jab, Jba) at several concentrations per timepoint.
Output Dataset:
The team delivered a complete dataset from the flux experiments carried out on rat aProximateTM Proximal Tubule Cell model.
Example dataset:
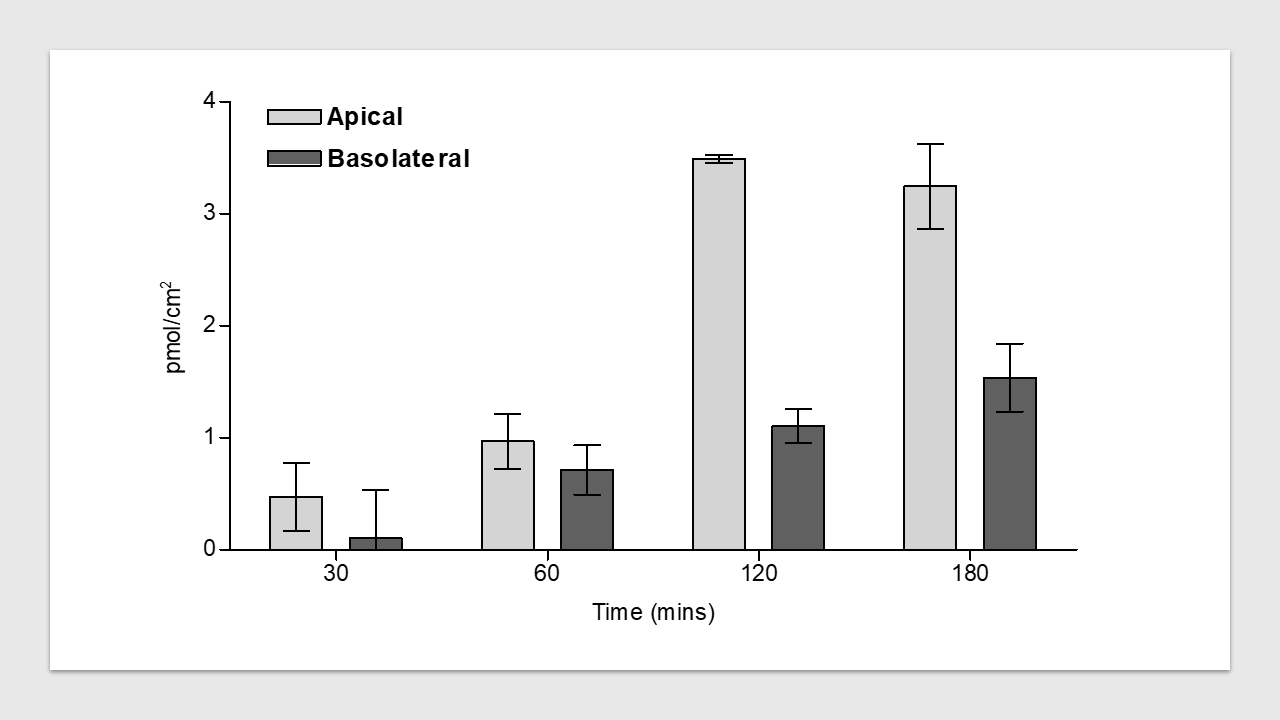
Figure 1: Initial rate of uptake of radioconjugated peptide across apical and basolateral membrane.
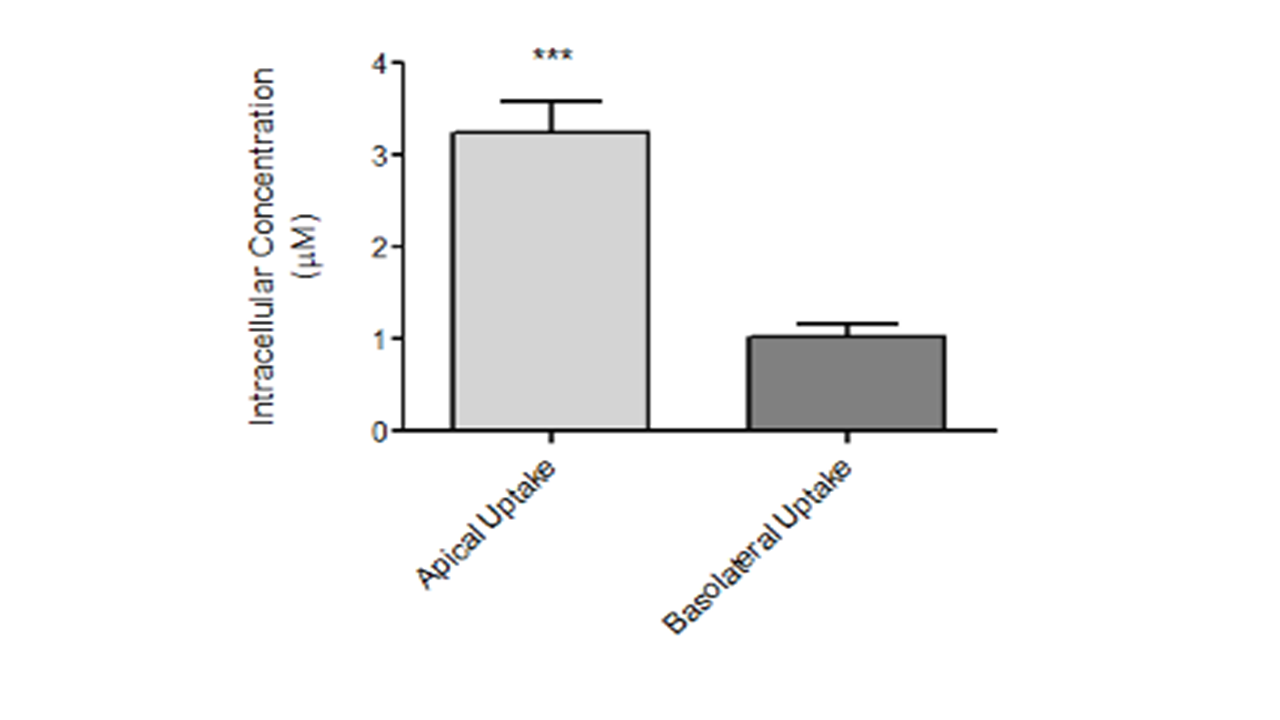
Figure 2: Intracellular accumulation of radioconjugated peptides across basolateral and apical membrane.
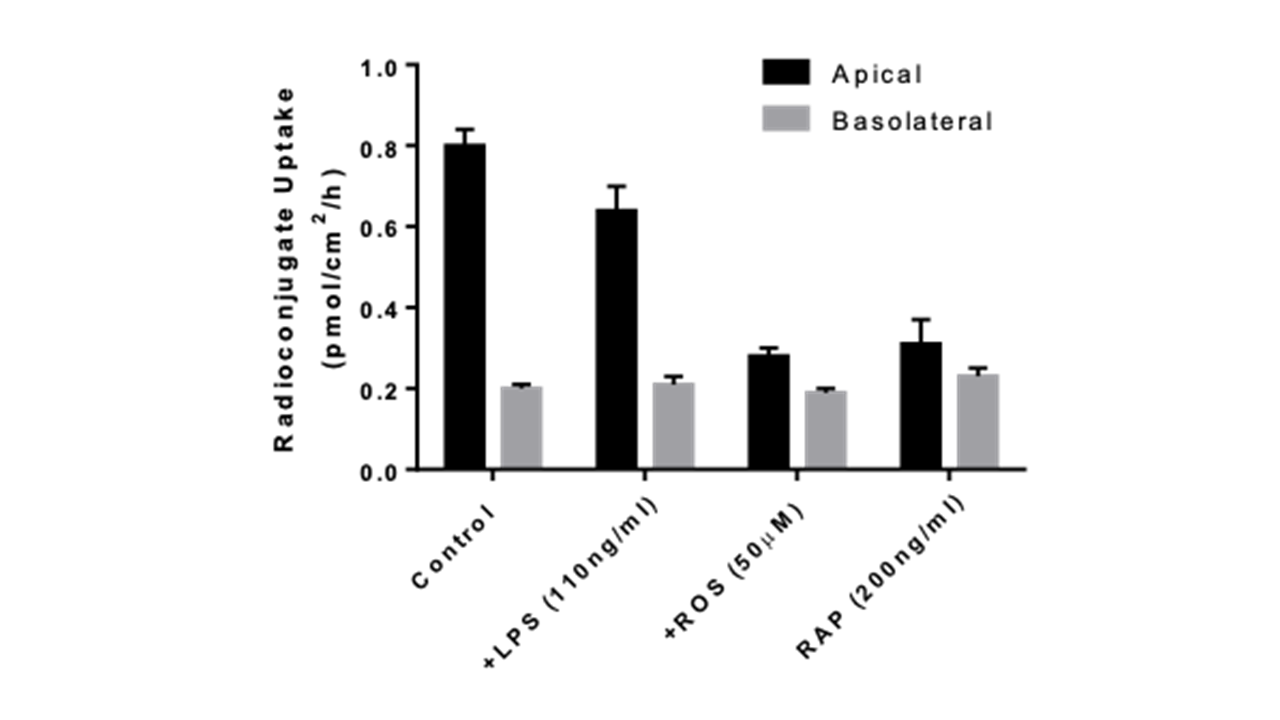
Figure 3: Uptake inhibition of radioconjugate by known inhibitors of Megalin/Cubilin (LPS, ROS and RAP).
Step 4:
Delivery of Results
We delivered a detailed report of the dataset which was shared digitally and discussed over a call.
The study gave clear functional uptake data, showing:
- Apical uptake of the radioconjugate peptide
- Intracellular accumulation of the radioconjugate peptide across mostly apical but also basolateral membrane
- Saturable uptake of the radioconjugate over time
- Partial role of Megalin/Cubilin in the uptake
Outcomes for the Client and the Project
The client was able to assess radioconjugate uptake and mechanism in the proximal tubule and was able to make an informed decision as to whether to progress their radioconjugate compound.