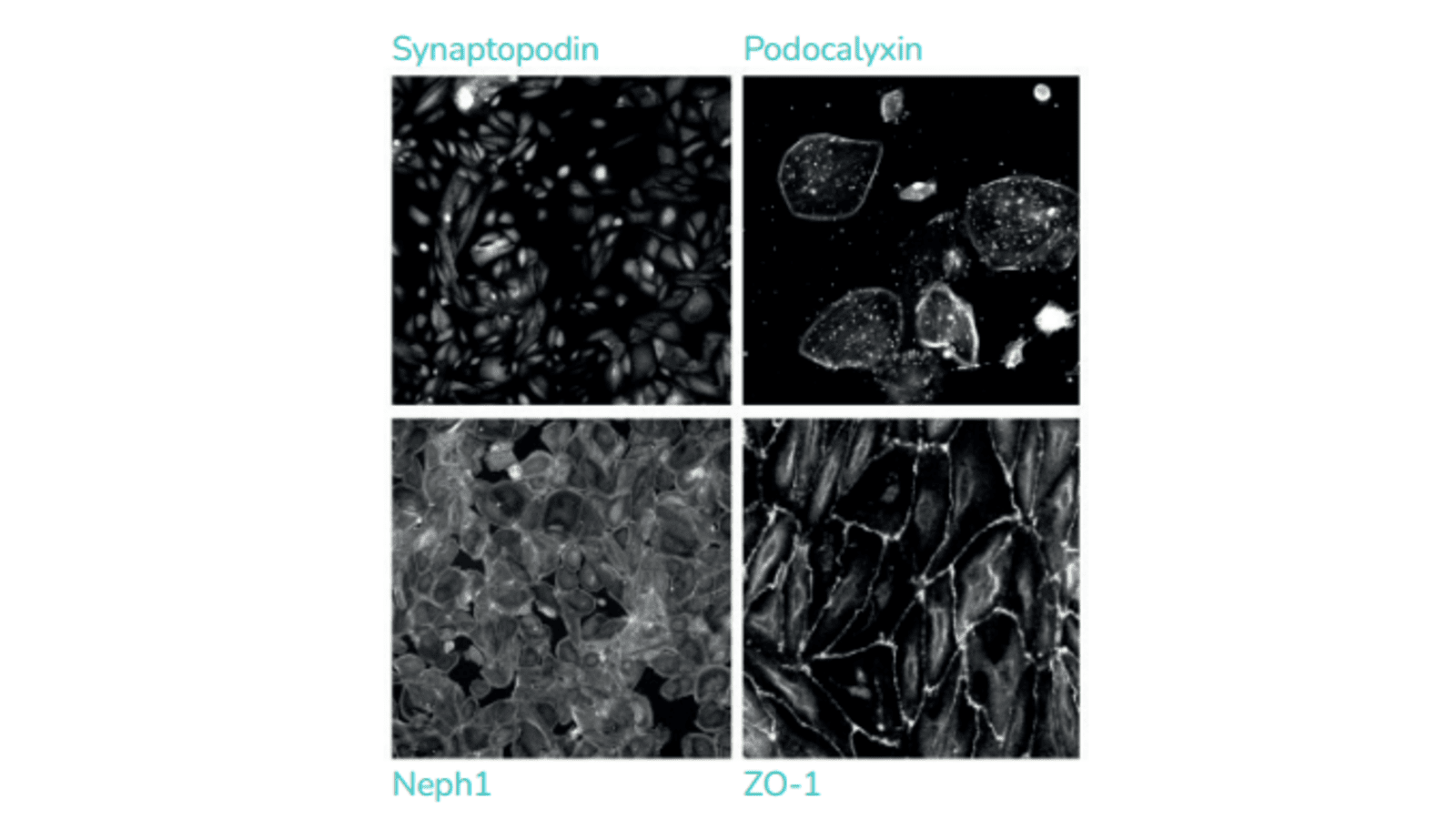
Evaluation of glomerular ADC toxicity in podocytes
Tissue
GlomerulusModels and Species
Kidney Toxicity ServiceThe ask
Our customer‘s goal was to evaluate the potential toxicity of a novel ADC test article compound on the human glomerular filtration barrier in vitro.
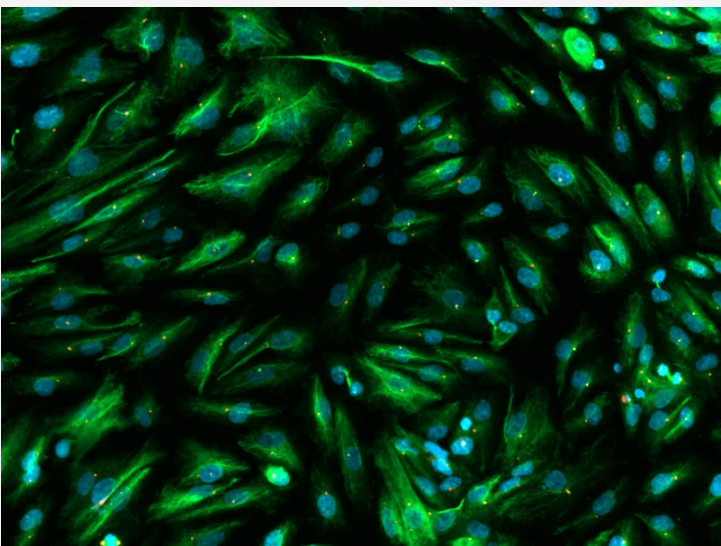
Our approach to developing a solution
We followed our standardised series of steps to develop a customised solution.
Step 1:
UNDERSTANDING THE PROBLEM
We clearly defined the problem and questions to be answered through in-depth discussion between the customer and our technical experts.
The customer wished to rapidly assess the glomerular toxicity of their novel test compound as part of their ADC drug development programme.
Taking all project specific factors into account, we suggested a two-step approach as being the most appropriate for their situation:
- Step 1: confirm the expression of the ADC target in our monolayer podocyte model.
- Step 2: carry out a defined set of in vitro studies to determine the impact of the test article compounds on the human podocyte monolayer integrity.
Step 2:
DEVELOPING A CUSTOMISED EXPERIMENTAL PLAN
We designed the specific experiments and presented the plan in our Statement of Work within 1 week.
Our experimental plan involved using human primary podocytes isolated from a single donor for each experiment.
For Step 1, we outlined qRT-PCR experiments to determine the expression of the test article target.
For the second set of experiments, we designed a plan to
- grow the podocytes as a monolayer on transwells
- monitor the barrier integrity using measurements of transepithelial electrical resistance (TEER) and permeability assays to 70 kDa FITC-dextran.
- assess cell health (viability) through measurement of ATP.
- use primary proximal tubules as a positive control.
Step 3:
PROJECT EXECUTION
Our scientists carried out the agreed experiments within 1 months providing regular updates to the customer
The study included the experimental phase, data processing, data analysis and presentation of a data summary.
DATASET produced:
We produced a total of 135 data points
- 2 test articles compounds each tested for, 6 concentrations,
- 1 positive control and 1 negative control
- TEER, FITC-dextran and ATP Assay for each of the above.
Each data point was carried out in triplicate.
Step 4:
RESULT DELIVERY
We delivered a detailed report with reliable dataset which was shared digitally and discussed over a call upon request.
Outcomes for the client
We delivered a robust dataset which clearly showed the concentration at which each ADC test article started to disrupt the podocyte monolayer integrity. Our analysis gave confidence to our customer to pursue their drug development program.