aProximate™ Proximal Tubule Assay-Ready Plates: Advancing Drug Research with Accurate In Vitro Models
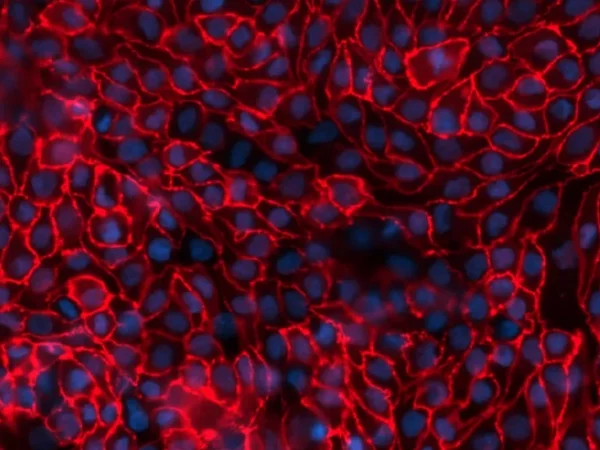
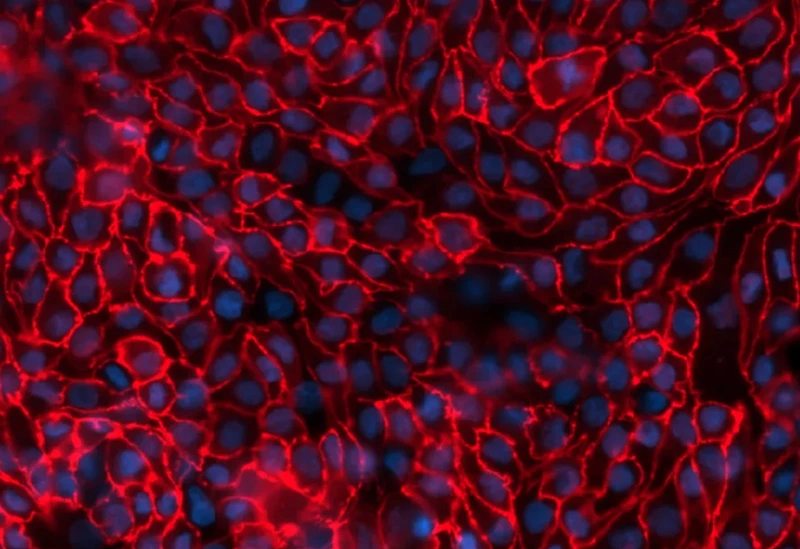
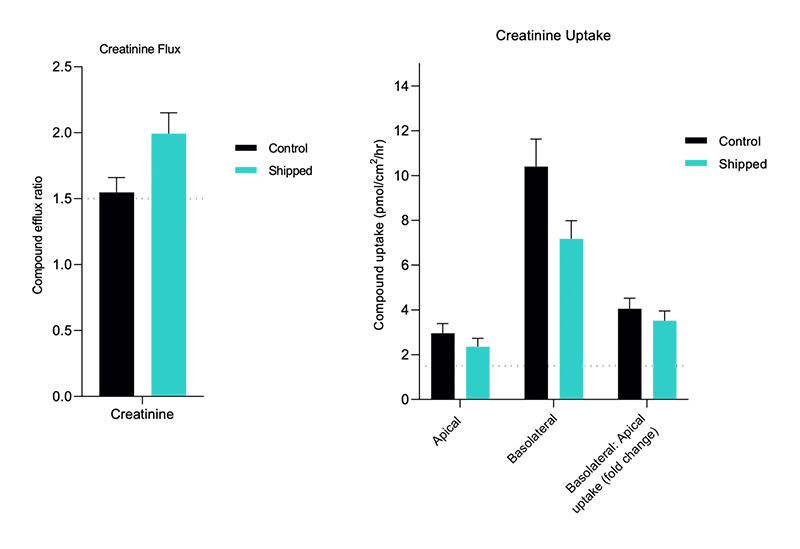
aProximate™ Proximal Tubule Assay-Ready Plates offer an innovative approach to drug research. providing an accurate in vitro model of the proximal tubule derived from fresh human kidney tissue. Unlike other primary cell models and cell lines, these unique assay-ready plates are freshly isolated from human donor kidney tissue, never frozen or passaged, ensuring unparalleled accuracy and reliability in your experiments. With worldwide shipping options, our assay-ready plates are accessible to researchers across the globe and delivered to you ready for your assays.
Features and benefits
- Accurately recapitulates the function of the proximal tubule
- Global accessibility
- Increased efficiency with high throughput
Don't miss out on our latest innovations: follow us on Linkedin