Molecular and cellular mechanisms of kidney disease modelling in vitro
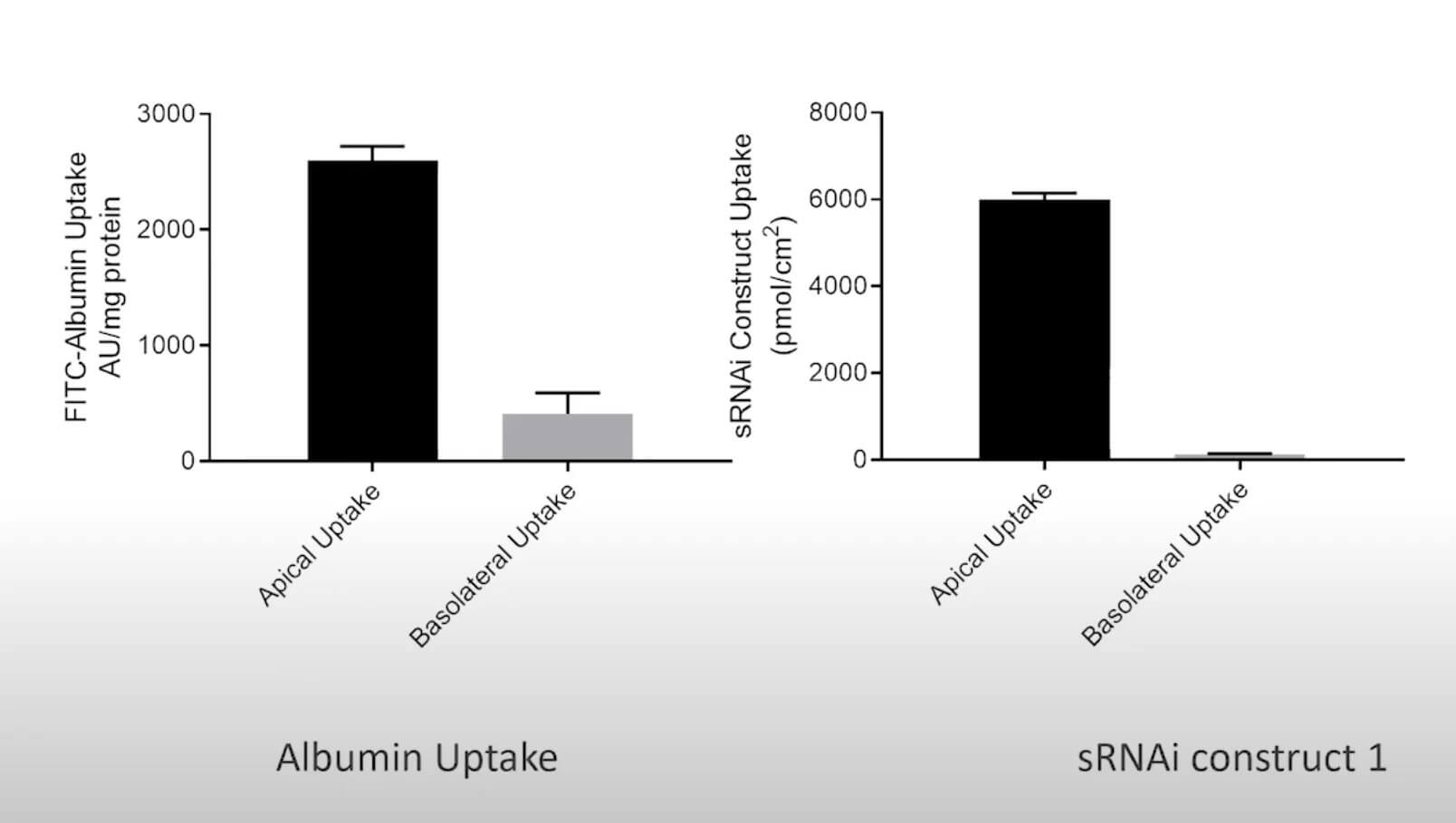
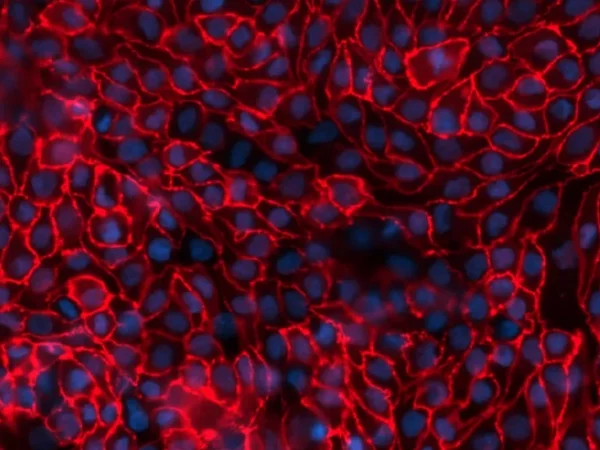
To study renal dysfunction and perform in vitro kidney disease modelling, a robust model, such as aProximate™, expressing all the transporters specialised in handling of drugs, solutes, and proteins is required. The Newcells platform is unique as it is highly predictive of in vivo outcomes. It also allows insights into disease mechanisms and assessment of therapeutic targets (e.g., renal transporters) of new drugs. aProximate™ has been used to study a range of diseases including chronic kidney disease (CKD), gout, and metabolic disorders.
Service outputs
- Apical to Basal (Jab) and Basal to Apical (Jba) flux
- Net transport
Don't miss out on our latest innovations: follow us on Linkedin