Light responsive retinal organoids for accurate prediction of clinical outcomes
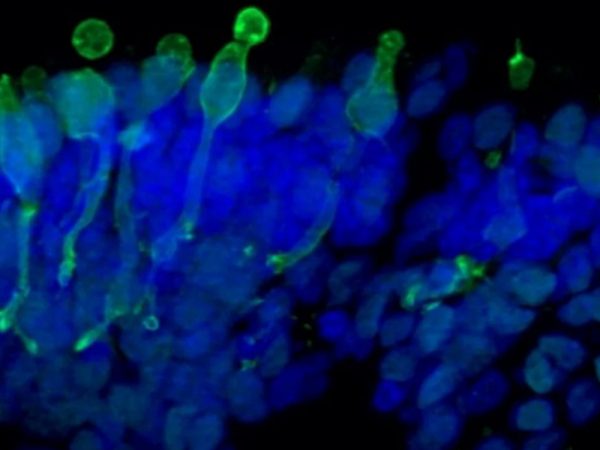
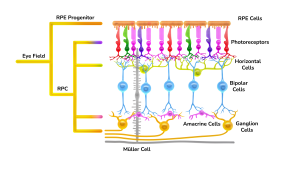
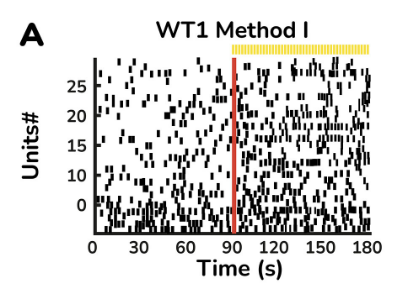
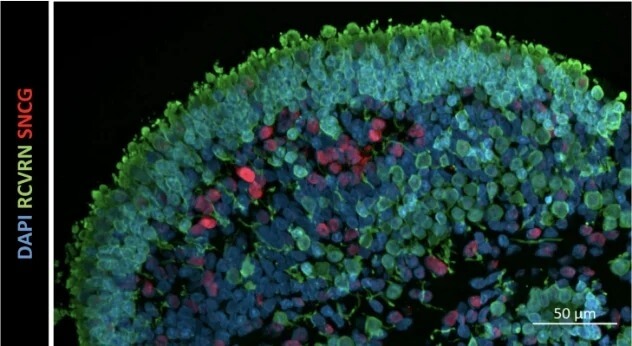
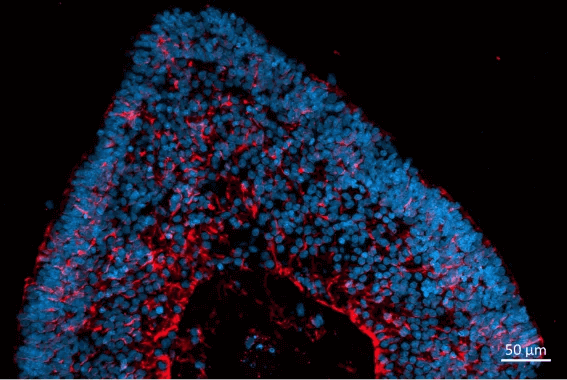
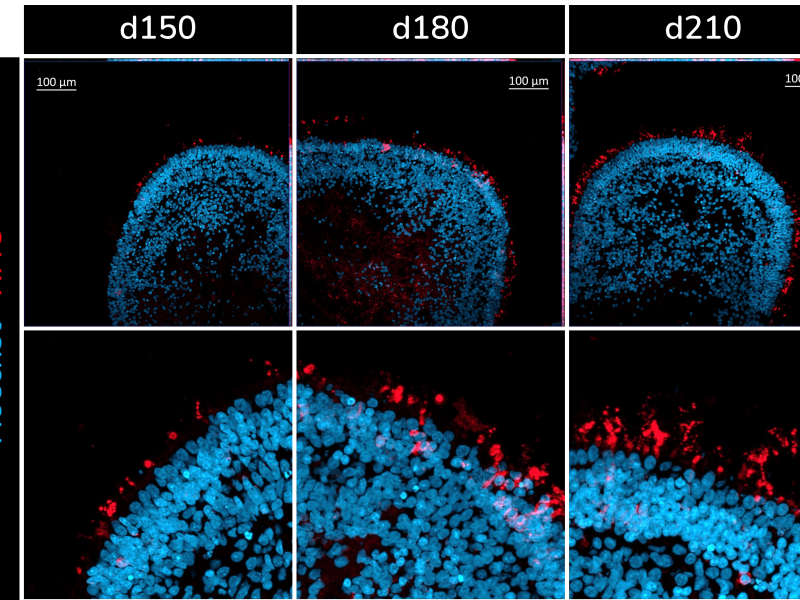
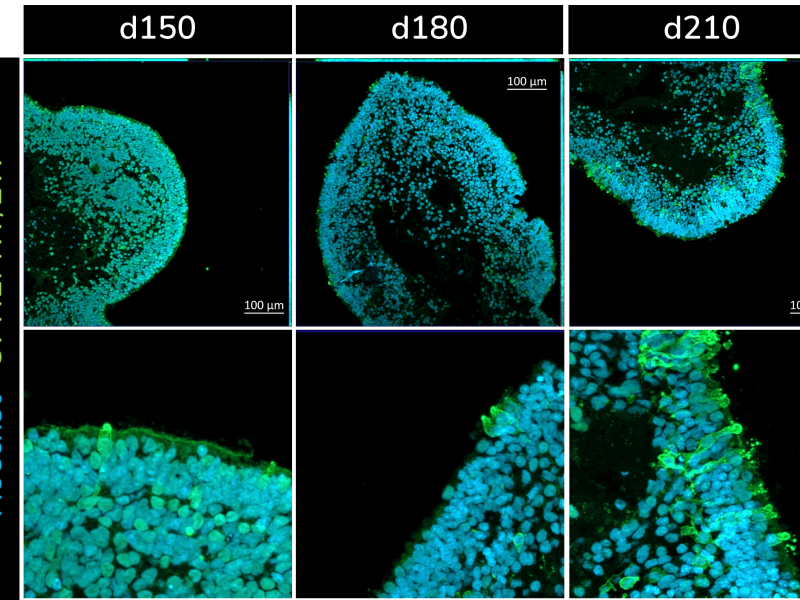
The retinal organoids recapitulate the complex structure of the human retina with laminar cell organisation mimicking embryonic development. They contain the outer photoreceptor segment of the retina that responds to light.
Available analytical readouts
- Immunofluorescence analyses
- mRNA quantification by RT-qPCR
- Transcriptomic analysis by single-cell RNA sequencing
- Cytotoxicity assays
- Cytokine release
- Flow cytometry
- Electron microscopy
Don't miss out on our latest innovations: follow us on Linkedin